-
Probing chiral interfaces by infrared spectroscopic methods
M. Bieri, C. Gautier and T. Bürgi
Physical Chemistry Chemical Physics, 9 (6) (2007), p671-685


DOI:10.1039/b609930k | unige:14763 | Abstract | Article HTML | Article PDF
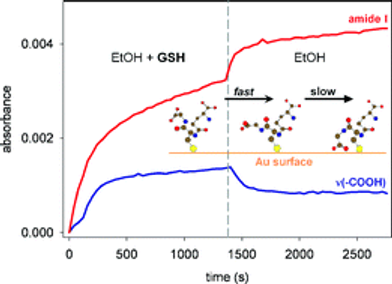
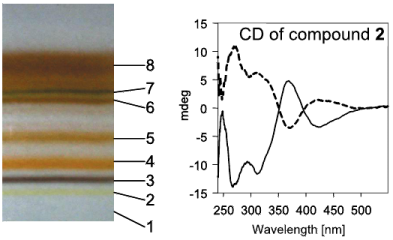
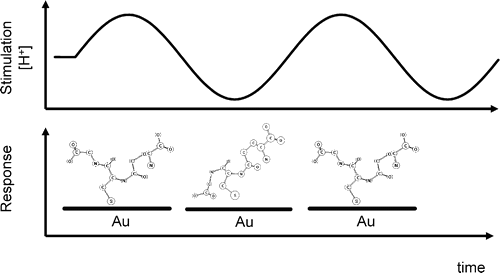
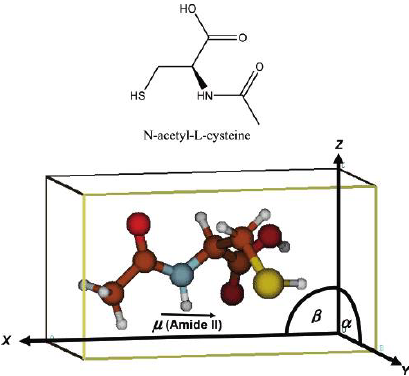
Biological homochirality on earth and its tremendous consequences for pharmaceutical science and technology has led to an ever increasing interest in the selective production, the resolution and the detection of enantiomers of a chiral compound. Chiral surfaces and interfaces that can distinguish between enantiomers play a key role in this respect as enantioselective catalysts as well as for separation purposes. Despite the impressive progress in these areas in the last decade, molecular-level understanding of the interactions that are at the origin of enantiodiscrimination are lagging behind due to the lack of powerful experimental techniques to spot these interactions selectively with high sensitivity. In this article, techniques based on infrared spectroscopy are highlighted that are able to selectively target the chiral properties of interfaces. In particular, these methods are the combination of Attenuated Total Reflection InfraRed (ATR-IR) with Modulation Excitation Spectroscopy (MES) to probe enantiodiscriminating interactions at chiral solid–liquid interfaces and Vibrational Circular Dichroism (VCD), which is used to probe the structure of chirally-modified metal nanoparticles. The former technique aims at suppressing signals arising from non-selective interactions, which may completely hide the signals of interest due to enantiodiscriminating interactions. Recently, this method was successfully applied to investigate enantiodiscrimination at self-assembled monolayers of chiral thiols on gold surfaces. The nanometer size analogues of the latter—gold nanoparticles protected by a monolayer of a chiral thiol—are amenable to VCD spectroscopy. It is shown that this technique yields detailed structural information on the adsorption mode and the conformation of the adsorbed thiol. This may also turn out to be useful to clarify how chirality can be bestowed onto the metal core itself and the nature of the chirality of the latter, which is manifested in the metal-based circular dichroism activity of these nanoparticles.